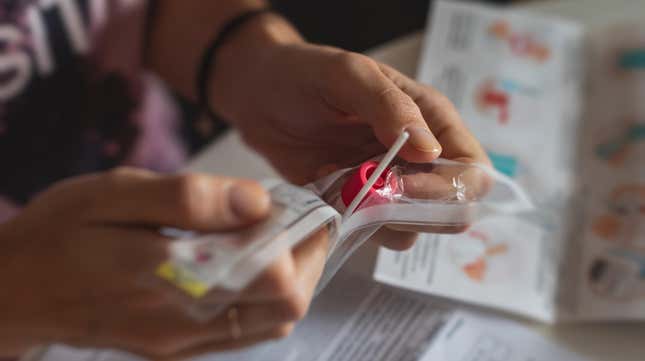
The last thing you’d expect when testing for a virus is to catch a bacterial infection in the process. But that could be the the case with some at-home COVID-19 tests distributed in the United States, according to a U.S. Food and Drug Administration recall. More than 500,000 of the tests were distributed to CVS drugstores and around 16,000 to Amazon, according to the BBC. The South Korean company, SD Biosensor, is voluntary recalling their kits in the U.S. and is asking consumers to stop using them and to dispose of them properly.
The test kits are a rapid antigen test that use a nasal swab to collect and test a sample. There is the potential risk of contracting a “potentially harmful bacteria” from the liquid inside the tube found in pouch number two in the test kits.
Which COVID test kits are recalled?
The name of the product is “Pilot COVID-19 At-Home Tests” (see an image here) and the recalled ones can be identified by the following lot numbers on the outside of the package:
- 53K38N1T1, 53K38N2T1, 53K38N3T1, 53K38N4T1, 53K38N5T1, 53K38P1T1, 53K38P2T1, 53K38P3T1, 53K41T5T1, 53K41X1T1, 53K41X2T1, 53K41X3T1, 53K4211T1, 53K4212T1, 53K4213T1, 53K4221T1, 53K4222T1, 53K4223T1, 53K4224T1, 53K4225T1, 53K4231T1, 53K4232T1, 53K4233T1, 53K4261T1, 53K4262T1, 53K4271T1, 53K4272T1, 53K4273T1, 53K4274T1, 53K4291T1, 53K4292T1, 53K42A1T1, 53K42A2T1, 53K42A3T1, 53K42E1T1, 53K42G1T1, 53K42G2T1, 53K42H1T1, 53K42H2T1, 53K42L1T1, 53K42L2T1, 53K4361AC, 53K4362AC, 53K4392AC.
According to the recall, direct exposure to the liquid in the tube, which can happen when handling the self-test, could lead to serious illness from bacterial infection. These include enterococcus, enterobacter, klebsiella, and serratia species, which include signs of fever, discharge, and red eyes, according to the BBC. They also claim no official reports of illness dealing with the recall has been reported.
The recalled kits were distributed by Roche Diagnostics to distributors and retailers across the U.S. and were available over the counter.
What to do if you have the recalled test kits?
If you have one of the listed lot numbers above, the FDA urges you not to use them and to discard them properly: Throw away the entire kit in the household trash and do not pour the liquid down the drain. If you make contact with the liquid inside the tube on pouch number two, make sure to flush with large amounts of water. If you see an irritation that persists, the FDA recommends you seek medical attention.
You can find more information about the recall from the company’s website or by calling the Roche Customer Support Center at 1-866-987-6243 and selecting option 1. If you have a side effect from the recall you want to report, you can do so here.

Comentarios recientes